Guardian AI-powered remote patient monitoring for the operating room
case study highlight
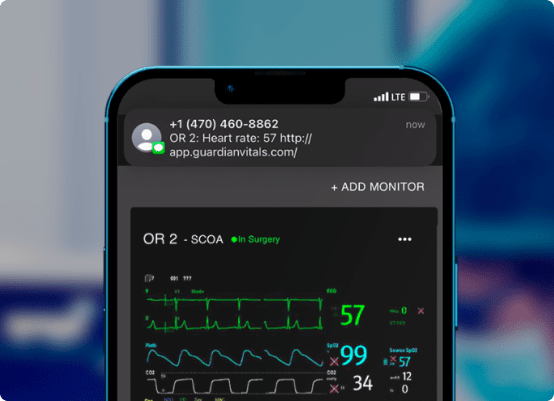
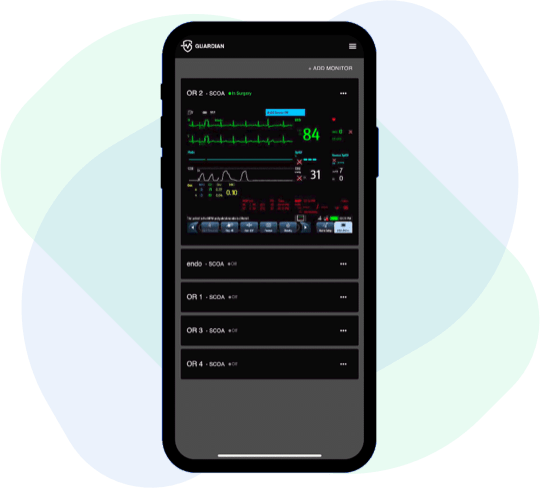
AI-powered remote patient monitoring for the operating room
Mobile app enables clinicians to monitor patient vital signs from their smartphone – multiple patients, one screen.