Blog Summary:
In our recent webinar with Chandler Tames from Rook Quality Systems, we highlighted the importance of human-centered design in medical device app development, early regulatory pathway identification to save time, and the value of thorough performance testing. Our Product Blueprint at Digital Scientists helps reduce risks by aligning research, prototyping, and validation. For more details, watch the full session.
I recently had the pleasure of co-hosting a webinar alongside Chandler Tames from Rook Quality Systems, where we explored the nuances of developing patient-centric medical device apps while navigating regulatory compliance. For those who couldn’t attend, I wanted to share some of the key takeaways that stood out from our discussion.
1. Human-Centered Design is Essential
One of the core principles we use at Digital Scientists is design thinking. This approach puts the patient at the center of the development process. In healthcare, it’s easy to get lost in the technical and regulatory complexities, but the patient’s journey is what truly matters. We need to design solutions that integrate seamlessly into their healthcare experience, considering not only the medical issue but also the relationships, tools, and information they rely on.
2. Early Regulatory Pathway Identification Saves Time
Chandler highlighted the importance of understanding and identifying regulatory requirements early in the development process. Whether your medical device app is categorized as Class I, II, or III by the FDA, this decision has a massive impact on your project timeline and resources. Tackling regulatory hurdles sooner rather than later can save a lot of headaches down the road.
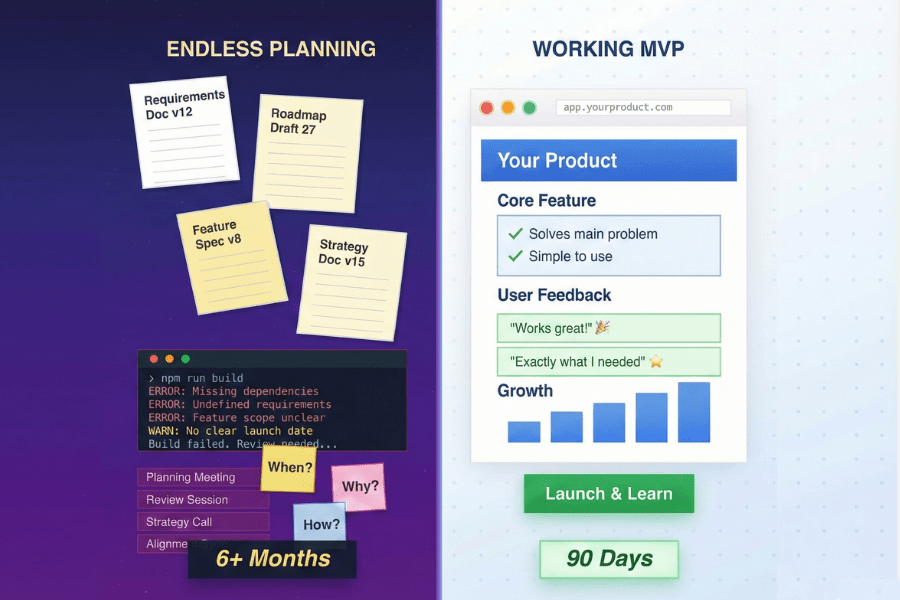
3. Our Product Blueprint Reduces Risk
At Digital Scientists, we’ve developed a process called the Product Blueprint. This 8–12 week method focuses on alignment, research, prototyping, and validation. By following this structure, we ensure that our solutions not only meet user needs but are also ready for development with minimal risk. This structured approach has helped us deliver successful products time and again.
4. Performance Testing is Crucial
Both clinical and non-clinical performance testing are non-negotiable steps in medical device app development. Thorough testing early on ensures compliance, functionality, and safety, which are critical to getting your product to market. It’s much better to catch issues early in the testing phase than to face delays later in the process.
Watch the Full Webinar
These are just a few highlights, but there’s so much more covered in the webinar, from regulatory pathways to in-depth performance testing strategies. If you’re developing a medical device app or interested in patient-centric design, I highly encourage you to check out the full session here. You’ll gain a deeper understanding of how to mitigate risks and ensure your product’s success.